Welcome to blog sawobakejul, in this post please see the contents then take the necessary information.
title : 67 510K) FLOWCHART
link : 67 510K) FLOWCHART
Hai, thank you for visiting this amazing site to look for 510k) flowchart. I really hope the data that appears could be useful to you


![flowchart 510k) Organization Umd And Chapter Ii 100 [ Administration ] flowchart 510k) Organization Umd And Chapter Ii 100 [ Administration ]](https://lh3.googleusercontent.com/blogger_img_proxy/AEn0k_uuocTbSv-YtLrpYkKY6z9aXLcay8a3_rkzIxM7MSqyQvfYCuh23Qg8zmXOpXqqZBRg3TW5kKYnPS3CM196LaR4TK6Dx8vVQphyqJ-wpF-bjkf-Oazg0BAek4wdBIjQIDEkZEjA48dJJF-VCjo1R1LcItbutE3u0lENgm0tZGv3cq1OgG2r=s0-d)



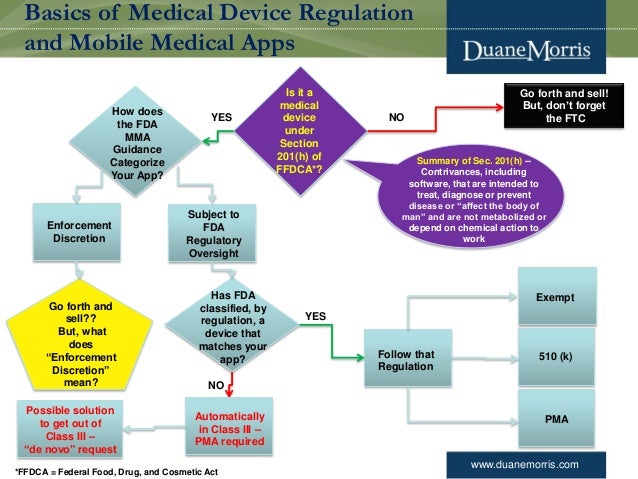
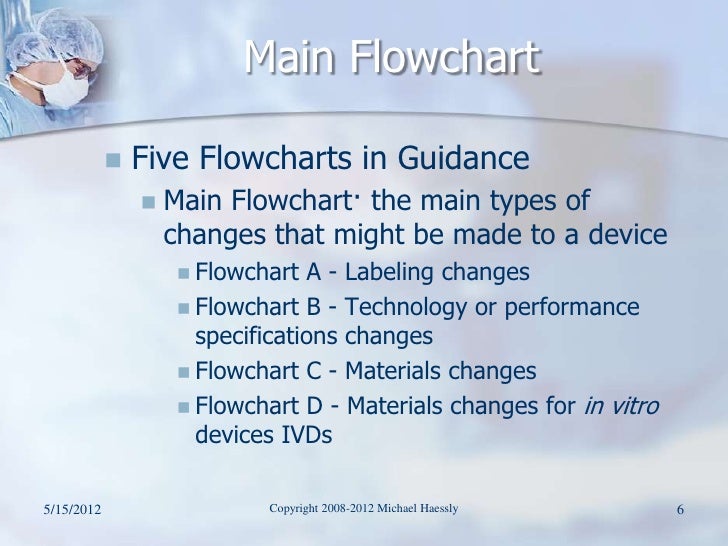

You are in the article 67 510K) FLOWCHART with url address https://sawobakejul.blogspot.com/2018/07/67-510k-flowchart.html
title : 67 510K) FLOWCHART
link : 67 510K) FLOWCHART
67 510K) FLOWCHART
510K) FLOWCHARTKagetsu Tohya Flowchart Create A Flowchart , Imaging3, Inc. (IGNG): Dominion DVIS is not De novo review , 100 [ Chapter Ii Organization And Administration ] Umd , Flowchart of the traditional DV Hop algorithm , Material Change What It Is and Tips for Dealing With It , 93/42/EEC(MDD) , Overview of FDA Regulation of Medical Devices , Deciding When To Submit A 510(K) For A Change To An , Guide on Class III MDD Medical Devices CE marking (mark , Define medical device software verification and validation , Medical Device Production Process Flow Diagram : 46 Wiring ,
Hai, thank you for visiting this amazing site to look for 510k) flowchart. I really hope the data that appears could be useful to you



Such is the article 67 510K) FLOWCHART
hopefully can be useful for the needy. Admin sawobakejul say Thank you for your visit.
You are in the article 67 510K) FLOWCHART with url address https://sawobakejul.blogspot.com/2018/07/67-510k-flowchart.html

0 Komentar untuk "67 510K) FLOWCHART"
Note: Only a member of this blog may post a comment.